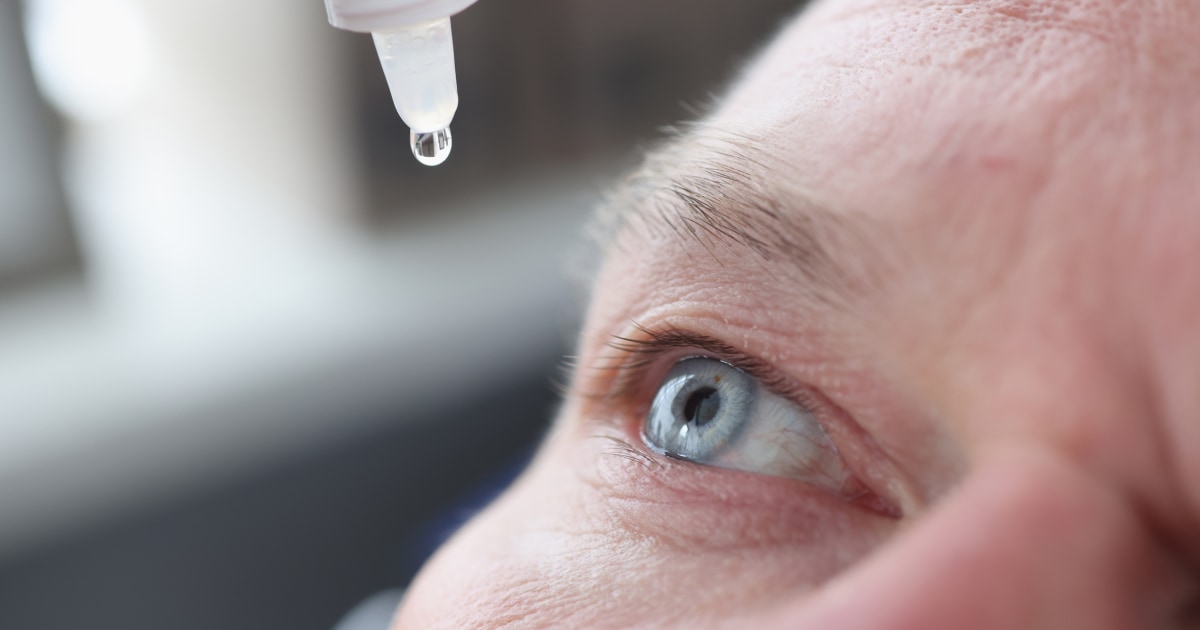
One person died and at least three others were left with permanent vision loss due to a bacterial infection possibly linked to a brand of over-the-counter eye drops, according to the Centers for Disease Control and Prevention.
Most of those affected reported having used without condoms EzriCare Artificial Tears before becoming ill, the CDC reported in a declaration dated January 20.
While the infections have not been definitively attributed to the eye drops, the CDC recommended that «patients immediately discontinue use of EzriCare Artificial Tears until epidemiologic investigation and laboratory testing is complete.»
So far, the CDC team has identified at least 50 people in 11 states with Pseudomonas aeruginosa, a type of bacteria resistant to most antibiotics. Cases have been reported in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, and Washington.
Most of the patients said they had used EzriCare Artificial Tears before they got sick.
Eleven developed eye infections, at least three of whom were blind in one eye. Others had respiratory infections or urinary tract infections. One person died when the bacteria entered the patient’s bloodstream.
It is not clear if the affected patients had underlying eye conditions, such as glaucoma or cataracts, that would have made them more susceptible. Symptoms of an eye infection include pain, swelling, discharge, redness, blurred vision, sensitivity to light, and the sensation of some type of foreign object being stuck in the eye.
Pseudomonas aeruginosa the bacteria are commonly found in water and soil and even on the hands of healthy people. Infections generally occur in hospital settings among people with weakened immune systems.
This type of bacteria is usually resistant to standard antibiotics.
«That’s the concern,» said Dr. Jill Weatherhead, an assistant professor of tropical medicine and infectious diseases at the Baylor College of Medicine in Houston. «Our standard treatments are no longer available» to treat this infection.
The drops under investigation are labeled as preservative-free. That is, the product does not contain anything that could prevent microbiological growth. The product could have been contaminated during the manufacturing process or when a person with the bacteria on their skin opened the container.
The CDC found the bacteria in vials of eye drops and is testing to see if that bacteria matches the strain found in patients.
As of Tuesday, EzriCare artificial tears had not been withdrawn from the market. They have been sold on Amazon and in stores like Walmart.